
Everett who helped to greatly increase Thomson's experimental range. About 1894 he acquired an excellent glassblower named E. He was very fumble fingered and had a tendancy to break things. 62 & 101) Measured the charge of an electron using oil droplets. 97-98) Thomson’s Plum Pudding Model, 1900 Electrons are dispersed in a uniform positive charge. Incidently, Thomson was a very unhandy person. Discovered electron’s charge to mass ratio: 1.76 x 108 C/g (p. The amount the cathode ray bent from the straight line using either the electric field or the magnetic field allowed Thomson to calculate the e/m ratio. This allowed him to use either electrical or magnetic or a combination of both to cause the cathode ray to bend. Thomson also could use magnets, which were placed on either side of the straight portion of the tube just to the right of the electrical plates. The two plates about midway in the CRT were connected to a powerful electric battery thereby creating a strong electrical field through which the cathode rays passed. The long glass finger (in the photo) projecting downward from the right-hand globe is where the entire tube was evacuated down to as good as a vacuum as could be produced, then sealed. This experiment was the first step of the JJ Thomson’s atomic theory. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. That glowing light particles were smaller than the atom. Thomson in 1897 announcing the discovery of the electron. JJ Thomson identified that the glowing light were the small particles. Th diagram below appeared in an article by J.J. It is about one meter in length and was made entirely by hand. Electrons are many thousand times smaller than the nucleus and negatively. Through his cathode ray experiments, Thomson also determined the electrical charge-to-mass ratio for the electron. by looking at angles of deflection with the Cathode Ray Tube, he calculated the charge to mass ratio of these particles by measuring the. The electron was the first subatomic particle ever discovered. Electrons are many thousand times smaller than the nucleus and positively charged. Thomson demonstrated that cathode rays, a new phenomenon, were made up of small negatively charged particles, which were soon named electrons.

Electrons are many thousand times larger than the nucleus and negatively charged. Thomson used the cathode ray tube to discover the electron and determine its negative. The image below of a CRT used by Thomson in his experiments. Thomson’s experiments with cathode ray tubes imply about the mass of an electron A. Identify and describe the five primary disciplines of chemistry. Only the end of the CRT can be seen to the right-hand side of the picture.
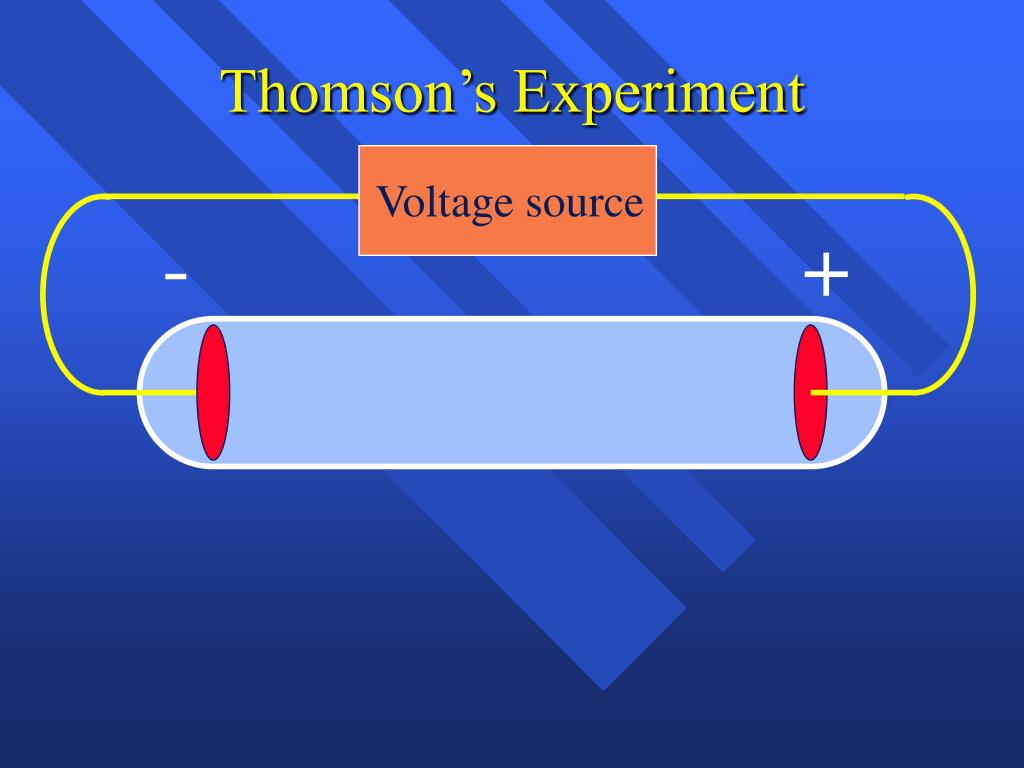

He received the 1906 Nobel Prize in Physics for this work. This was the first discovery of subatomic particles, which came to be called electrons. Thomson and a cathode ray tube from around 1897, the year he announced the discovery of the electron. Thomson discovered that the mass of the particles in cathode rays was 1800 times lighter than hydrogen, the lightest element. Thomson used results from cathode ray tube (commonly abbreviated CRT) experiments to discover the electron.


 0 kommentar(er)
0 kommentar(er)
